Master of Clinical Research

March 31, 2026
May 11, 2026
Clinical research is advancing at an unprecedented speed.
This 100% online clinical research program has been awarded esteemed academic accreditation and offers a multidisciplinary curriculum taught by faculty across the Colleges of Nursing and Pharmacy. Course offerings include the science and practice of research methods, medical product regulation, research ethics, pharmacology, statistics, project management, and research operations and quality management.
This is an applied program using experiential learning. You will add real-world course products to demonstrate acquired clinical research competencies in your Master of Clinical Research ePortfolio. As a graduate of this program, you will have acquired the skills and knowledge necessary to conduct, manage and regulate clinical trials. Applicants with any undergraduate degree are welcome to apply. Previously, students with degrees in the social sciences, nursing, public health, business, biology, and other health-related disciplines have successfully been admitted to this program. Admission to this program is available each autumn, spring, and summer semester. Students can complete this program in 1 to 3 years.
Learn more about how Ohio State is preparing the next generation of clinical research professionals.
*Not yet ready for a master’s program? Our online Clinical Research Management certificate may be the right fit. The graduate certificate program can be completed in two semesters, and all courses can be transferred to the master’s degree.
Frequently Asked Questions
Here are some frequently asked questions and answers we hope you find helpful. Please know that our team of Enrollment Advisors are always ready to connect and answer any questions that are specific to your unique needs.
Module lectures are recorded either by your instructors or from experts in the field, and you can view and participate at your convenience.
Yes! The Master of Clinical Research degree program was awarded the prestigious accreditation from the Commission of Accreditation on Allied Health Education Programs (CAAHEP) in 2023. The Ohio State University is accredited by the Higher Learning Commission (HLC) of the North Central Association of Colleges and Schools (NCA). Also, our program has been developed to meet the competency standards adopted by the Consortium of Academic Programs in Clinical.

The Master of Clinical Research classes use Carmen (Canvas), Ohio State’s learning management system. For each of the classes in which you enroll, the information will be posted in Carmen, including a syllabus and course calendar with due dates. Assignments are typically due by Sunday at midnight and students are encouraged to use time management when taking multiple courses. We recommend you login daily to ensure you’re keeping up with classes.
Commission on Accreditation of Allied Health Education Programs (CAAHEP)

Master of Clinical Research degree program awarded prestigious accreditation
Online program the second of its kind in the U.S. to earn academic accreditation
The Commission on Accreditation of Allied Health Education Programs (CAAHEP) has awarded its prestigious academic accreditation to the online Master of Clinical Research degree program. This is only the second such program across the United States to earn this accreditation.
Related Content
Related Content

Why Clinical Research is a Fast-Growing Career Field & How to Get Started

Charting Success: Patrick Rowan’s Journey to Clinical Research Leadership

What Can You Do With a Biology Degree?
Academic Calendar
For the convenience of online students, multiple start dates are offered during the academic calendar year for the online Master of Clinical Research program.
Academic Calendar
For the convenience of online students, multiple start dates are offered during the academic calendar year for the online Master of Clinical Research program.
Admissions Criteria
To be eligible for admission to the Master of Clinical Research program you must have the following:
You may have a bachelor’s degree in any area. Your degree must be from an accredited bachelor’s program. You may apply to the Master of Clinical Research program while your bachelor’s degree is still in progress, but you must receive the degree prior to beginning enrollment in the master’s program. Minimum of a 3.0 cumulative GPA on a 4.0 scale in the last degree earned that is relevant to the program of study.
*GPAs are reviewed by Ohio State’s Graduate and Professional Admissions Office. Applicants whose GPAs fall below 3.0 may still apply and be considered for admission.
How to Apply
Students must complete the following steps outlined below by the application deadline. Most applicants may review the status of their application any time at appstatus.osu.edu. Failure to complete all required steps of this application process by 11:59 pm ET on the day of the posted deadline will result in your application not being considered for admission, with no exceptions.
Submit the application online
You can complete an application online for the next available cohort at The Ohio State University Graduate and Professional Admissions office’s website. At the time you apply, you must select from either the Clinical Research Management or Regulatory Affairs specialization. This selection is made within the online application. A non-refundable $60 fee is required at the time of submission of the application
Submit official transcripts
To complete your application and verify your degree and undergraduate GPA, you must submit transcripts from all educational institutions you have attended.
When applying:
- You may upload scanned copies of official transcripts, diplomas, or degree certificates to the online application system. Note: You do not need to submit transcripts for coursework or degrees earned from The Ohio State University.
- These scans help form a complete application packet, but they do not replace the need for official documents.
What is not accepted:
- Advising reports
- Unofficial transcripts or scans labeled “UNOFFICIAL”
- Documents with a URL across the top
- Transcripts missing the institution’s name, seal, stamp, or GPA
These types of documents will not be used for degree verification or GPA calculation.
Exception: You do not need to submit transcripts for coursework or degrees earned from The Ohio State University.
Complete and submit all additional materials
Additional materials needed to apply include the following. You may submit the first two documents at the time of application or after by way of the Admissions Uploader. The recorded online video and letters of recommendation must be submitted by the application deadline.
- Three letters of recommendation
- Current resume or CV
- Purpose and goals statement
International applicants only – Test of English Proficiency
Details regarding what English Proficiency tests are acceptable, the scores required, and how to submit scores may be found on The Ohio State University Graduate and Professional Admissions office’s website.
Current or former Ohio State graduate students – Complete a supplemental application
If you are currently or have been previously enrolled in a degree granting graduate program at The Ohio State University for any length of time, you would be considered a Graduate Intra-University Transfer Student. Be sure to choose the appropriate application for “current or former” Ohio State students after following the Apply link found here. In addition to that online application, you must complete a Supplemental Application. This separate application must be submitted to the College of Nursing directly at CON-gradrecords@osu.edu or can be uploaded to your application after submission by way of the Admission Uploader. Choose any of the following options to access this application:
- MS Word Document: Supplemental MCR Application (To download, select “Save”)
- PDF: Supplemental MCR Application
Admission Timeline & Deferrals
Please ensure all the required materials listed above are submitted by the application deadline to be considered for admission. It may take up to five business days for the status of materials to be updated on your application status webpage.
*Please note that deferring admittance is not an option. If you are unable to start your studies in the term for which you applied, you will need to reapply in the future.
In accordance with the non-discrimination policy of The Ohio State University and the College of Nursing, we strictly prohibit any discrimination based upon age, color, ethnicity, race, sexual orientation, gender, gender identity, national origin, religion, pregnancy, or veteran status in its application, admission, or enrollment practices.
Ohio State is committed to treating applicants fairly and with dignity and respect. Please review Ohio State’s new post-admissions policy.

The Ohio State University participates in the State Authorization Reciprocity Agreements (SARA).
SARA is a national initiative that increases student access to distance education courses and programs while maintaining compliance with state regulations. Institutions participating in SARA can offer educational opportunities in all 49 SARA member states, the District of Columbia, the U.S. Virgin Islands and Puerto Rico without seeking individual approval in each state.
California is not a SARA member state, however, OSU may offer online courses and programs to students located in California under the California Private Post-Secondary Act of 2009.
The Application Process
Once you understand your program’s admission criteria, please note the application deadline. You’ll need a quiet space and a variety of materials for your application. To learn more, please see our Admissions page for the full process. Ready to Apply? Find your application here.
Career Outlook
The career outlook for graduates of Ohio State’s Master of Clinical Research is exceptionally promising. As the demand for clinical trials and healthcare innovations continues to grow, professionals with advanced expertise in clinical research are in high demand. The College of Nursing clinical research program supports growing industry need and prepares students to excel in professional roles on clinical research teams. Graduates can pursue diverse roles such as clinical research coordinators, clinical trial managers, regulatory affairs specialists, and clinical research associates in leading pharmaceutical companies, biotechnology firms, academic institutions, and government agencies. With a strong foundation in clinical trial design, data management, and regulatory compliance, graduates are well-prepared to lead and contribute to groundbreaking research that advances medical science and improves patient care. The program equips students with the skills and knowledge necessary to excel in a rapidly evolving field, making them valuable assets in the quest for new treatments and medical advancements.
Whether you’re looking to grow in your current career or make a career change altogether, Ohio State’s online programs can help you achieve your goals. Learn what the outlook is for your current or next career move using O*Net’s My Next Move tool.
Top Occupations by Median Income
What They Do
Apply knowledge of health care and database management to analyze clinical data, and to identify and report trends.
Work Activities
Design and validate clinical databases, including designing or testing logic checks. Process clinical data, including receipt, entry, verification, or filing of information. Generate data queries, based on validation checks or errors and omissions identified during data entry, to resolve identified problems.
Wage Range
- Entry Level: $63,650
- Mid Level: $112,590
- Senior Level: $194,410
Job Outlook
Bright
Projected Growth
36%
Related Careers
- Bioinformatics Technicians
- Clinical Research Coordinators
- Data Scientists
- Health Informatics Specialists
- Social Science Research Assistants
Job Sectors
- English Language
- Computers and Electronics
- Customer and Personal Service
- Mathematics
- Medicine and Dentistry
What They Do
Perform complex medical laboratory tests for diagnosis, treatment, and prevention of disease. May train or supervise staff.
Work Activities
Conduct chemical analysis of body fluids, including blood, urine, or spinal fluid, to determine presence of normal or abnormal components. Analyze laboratory findings to check the accuracy of the results. Operate, calibrate, or maintain equipment used in quantitative or qualitative analysis, such as spectrophotometers, calorimeters, flame photometers, or computer-controlled analyzers.
Wage Range
- Entry Level: $38,020
- Mid Level: $61,890
- Senior Level: $97,990
Job Outlook
Average
Related Careers
- Cytogenetic Technologists
- Cytotechnologists
- Histology Technicians
- Histotechnologists
- Medical and Clinical Laboratory Technicians
Job Sectors
- Biology
- Medicine and Dentistry
- Customer and Personal Service
- Chemistry
- English Language
What They Do
Plan, direct, or coordinate production activities of an organization to ensure compliance with regulations and standard operating procedures.
Work Activities
Develop regulatory strategies and implementation plans for the preparation and submission of new products. Review all regulatory agency submission materials to ensure timeliness, accuracy, comprehensiveness, or compliance with regulatory standards. Direct the preparation and submission of regulatory agency applications, reports, or correspondence.
Wage Range
- Entry Level: $68,860
- Mid Level: $136,550
- Senior Level: $227,590
Job Outlook
Bright
Projected Growth
5.7%
Related Careers
- Clinical Data Managers
- Compliance Managers
- Environmental Compliance Inspectors
- Management Analysts
- Regulatory Affairs Specialists
Job Sectors
- English Language
- Law and Government
- Administration and Management
- Biology
- Education and Training
What They Do
Conduct research using bioinformatics theory and methods in areas such as pharmaceuticals, medical technology, biotechnology, computational biology, proteomics, computer information science, biology and medical informatics. May design databases and develop algorithms for processing and analyzing genomic information, or other biological information.
Work Activities
Develop new software applications or customize existing applications to meet specific scientific project needs. Communicate research results through conference presentations, scientific publications, or project reports. Create novel computational approaches and analytical tools as required by research goals.
Wage Range
- Entry Level: $54,500
- Mid Level: $93,330
- Senior Level: $159,780
Job Outlook
Bright
Projected Growth
5.6%
Related Careers
- Bioinformatics Technicians
- Biostatisticians
- Data Scientists
- Geneticists
- Molecular and Cellular Biologists
Job Sectors
- Biology
- Computers and Electronics
- Mathematics
- English Language
- Chemistry
National occupational information in Ohio State Online’s Career Outlook tool is sourced from O*NET Online and the U.S. Bureau of Labor Statistics (BLS). The median annual wage displayed to the right of each occupational title above is based on the BLS Employment Projections program. Outlook and percent change indicate projected growth or decline over the next 10 years.
Mapping the Pathway to Take Control of Your Clinical Research Career
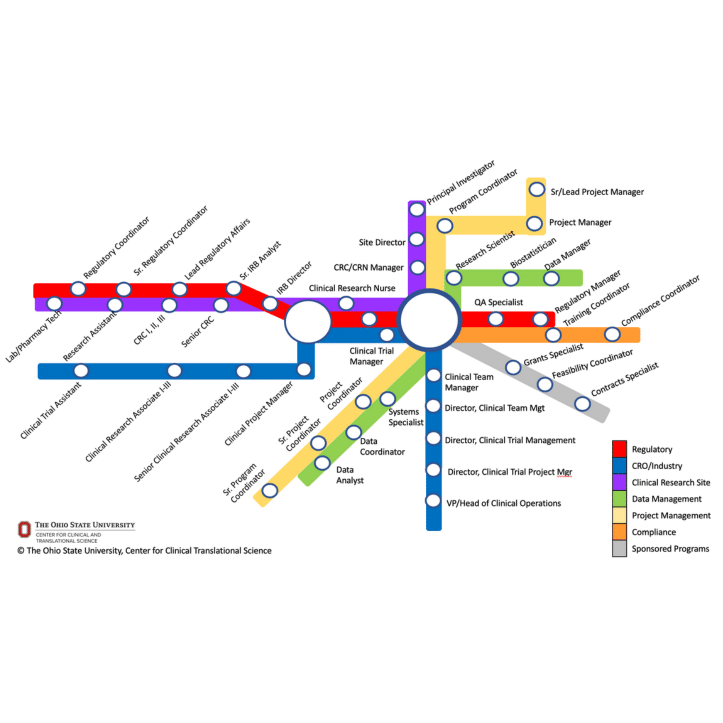
Authored By: Jessica Fritter, MACPR, ACRP-CP; Carolynn Jones, DNP, MSPH, RN, CRN-BC, FAAN
The Association of Clinical Research Professionals (ACRP)
“If you have ever traveled by metro, you know that you may only have limited options when you first get on at a nearby station, but you can eventually choose from among many different routes offered at various intersecting hub stations…. We wanted to illustrate that there are career pathways based on roles or employers, and that there are also opportunities for switching pathways (e.g., from the red line to the blue line) to reach your ultimate destination. While the exact job title may vary between organizations and institutions, this map can provide insights into the variety of roles one can start and grow beyond in your clinical research professional career.”
Now is the time to fix the clinical research workforce crisis

Authored By: Freel et al, 2023
National Library of Medicine
“The clinical and translational research enterprise is recognized by many as the ‘evidence generation system.’ While there have been several calls to revolutionize this enterprise to more effectively deliver the fruits of biomedical science to patients and society, significant issues across the clinical research workforce are pervasive. Perhaps the most visible sign is the widening gap between supply and demand for competent staff. Underpinning this, is a perfect storm of complex issues. Now reaching crisis point, this problem is far bigger than a staffing issue and ultimately jeopardizes the ‘engine’ of drug and device development. With the current perilous state of the workforce, proposed enterprise fixes are likely to languish far out of reach, given that even ‘business as usual’ is under threat. In fact, a glaring disconnect is evident between the visionary discourse on how to revolutionize the clinical research enterprise and the sober recognition that operationalization of any such vision rests on the shoulders of a workforce that’s in dire straits…”
Curriculum
Experience top-tier curriculum in our online Master of Clinical Research program. Courses are available online asynchronously, meaning there are no mandatory “live” online lectures or discussions so you can attend classes on your own time. Module lectures are recorded either by your instructors or from experts in the field, and you can view and participate at your convenience.
Function of clinical research in medical product development and the regulatory process of new medical products. Laws and regulations concerning the development, testing, commercialization, and total product life cycle for medical products. Regulations governing the conduct of clinical research, including study sponsors, investigators, and Institutional Review Boards.
Study of research design and methods used in clinical research. Measurement issues, bias and confounding, statistical considerations, evaluation of published clinical research designs, and protocol and proposal development.
Introduction to the principles of biostatistical methods used in biomedical research. Analysis of clinical and preclinical research data and interpretation of statistical results in biomedical studies.
Comprehensive investigation of pharmacovigilance initiatives and pharmaceutical safety regulation. Pharmaceutical risk management in premarket testing and development, recognition of safety signals, post-approval experience, drug production, risk mitigation, and administration of pharmaceuticals.
Concepts and application of total quality management for federal regulation of medical product development including drugs and medical devices
This course overviews principles underlying drug action, including an investigation of current treatments for a variety of common diseases. In addition, this course will implement activities that emphasize the ethical aspects and implications of a variety of drug therapies.
Fundamental principles of clinical research operations from study site selection to study closure from the perspective of sponsors and clinical research sites including an introduction to database design, management, quality assurance, and reporting for site and sponsor operations.
Concepts and policies for the responsible conduct of research (RCR), Institutional Review Boards and dissemination of findings will be introduced.
The Clinical Research Management Specialization focuses on the management of systems and processes in the conduct of clinical trials to prepare graduates to lead complex national and international clinical research operations. Graduates of this track will have attained the skills to work as Clinical Research Coordinators (CRCs) or Clinical Research Associates (CRAs) representing the study sponsor or investigation site and to advance their careers as clinical research administrators.
In addition to the core coursework, students in the Clinical Research Management Specialization will complete the following courses:
Required specialization courses:
MCR 7599 Culminating Project in Clinical Research
MCR 7404 Project Management for Healthcare and Clinical Research
MCR 7481 Data Management and Informatics in Clinical Research
MCR 7402 Economic Evaluation of Healthcare Interventions (cost-effectiveness studies)
PHR 7570 Pharmaceutical Safety & Risk Management
This specialization emphasizes the assurance of the safe and effective medical product development and use throughout the product life cycle through regulatory strategy, oversight and technical writing. Graduates from this track will have the skills to interpret FDA and international agencies’ regulations and guide regulatory strategy and operations. Regulatory compliance professionals work in many settings, including government, industry, clinical research organizations and academic institutions.
In addition to the core coursework, students in the Regulatory Affairs Specialization will complete the following courses:
Required specialization courses:
MCR 7460 Regulatory Strategy, Writing and Leadership
PHR 7570 Pharmaceutical Safety & Risk Management
MCR 7572 Federal Regulations of Medical Products
PHR 7580 Principles of Safety Pharmacology
MCR 7599 Culminating Project in Clinical Research
Understanding Online Course Types
As you research the right online program for you, you likely will come across the terms “asynchronous” and “synchronous.” Learn what these terms mean and how they’re important to consider when understanding how a program will fit into your life.
Learn More

Message from the Director
We provide students with the knowledge and real-world, hands-on skillsets that enable success in clinical research careers. When you join our program, you’ll become part of a collaborative and supportive community led by devoted faculty, staff and students. In addition, the flexibility of the program allows it to be accessible to most working professionals. The MCR program admits students three times per year. As working professionals, the majority of students (82%) graduate in one to two years through our full-time or part-time options. Our students have contributed to improvements in the clinical research enterprise.
We are pleased to announce that in May 2023 our program became accredited by the Commission on Accreditation of Allied Health Education Programs (CAAHEP). It was the second graduate program to achieve this recognition.

Jessica Fritter, DHSc, MACPR, ACRP-CP
Dr. Jessica Fritter is dedicated to strengthening the clinical research workforce through teaching, research, and student engagement. She develops and teaches key courses in clinical trials, data management, and project management. Her research focuses on workforce development, diversity, and education. Dr. Fritter holds a Doctor of Health Sciences from Eastern Virginia Medical School, a master’s from Ohio State, and a bachelor’s from UNC Wilmington.
Ester Chipps PhD, RN, FAAN
Clinical Professor
Lauren Hill MSN, RN
Clinical Instructor of Practice
Tia Patterson PhD, CCRP
Clinical Assistant Professor of Practice
Samantha Sharpe MD
Clinical Assistant Professor of Practice
Testimonials
Don’t just take our word for it. Hear what MCR alumni are saying about Ohio State Online’s Master of Clinical Research program.
Testimonials
Don’t just take our word for it. Hear what MCR alumni are saying about Ohio State Online’s Master of Clinical Research program.

"You can learn a lot while working in the field, but you won’t learn it to the extent that the MCR program will teach you."

"I’ve made lasting relationships. I still meet my former classmates to discuss processes, improvements and research that we are conducting."

“The online structure made it easy to balance full-time work with graduate study—and the faculty support has been incredible.”
Tuition fees are subject to change. The table above serves as a guide and not an official bursar’s bill. Full-Time costs are total tuition costs per semester.
Scholarships
Scholarships offered through the College of Nursing are made available for application each spring semester. Thanks in large part to generous private donors, the College of Nursing has a number of scholarships available. Continuing students are invited to submit the scholarship application to be considered for need-based and/or merit-based scholarships. These funds are limited and preference is given to full-time students who are continuously enrolled.
Financial Aid Resource
Financial Aid Resource
Related Articles
Related Articles

How to ask your employer for tuition reimbursement
How to Pay for Your Online Program

Tips for Online Learning from Ohio State Students and Faculty
Get Started
Connect with a knowledgeable Enrollment Advisor who can help answer your questions and explain different aspects of the more than 80 online degrees and certificates offered at Ohio State. They are here to help you on your education journey.


